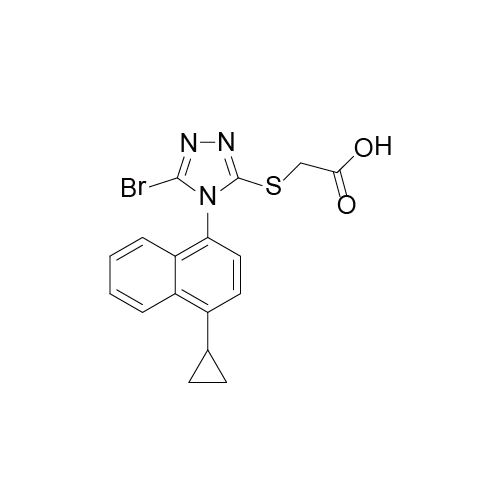
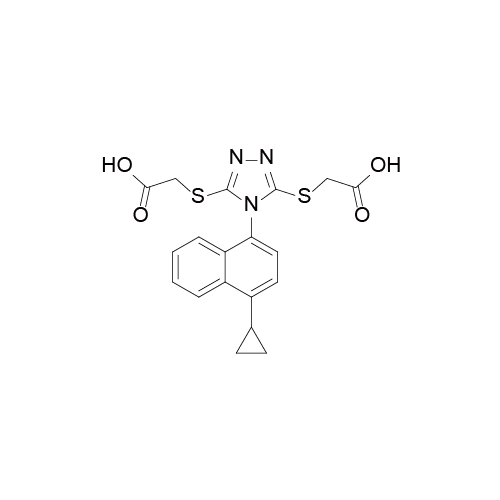
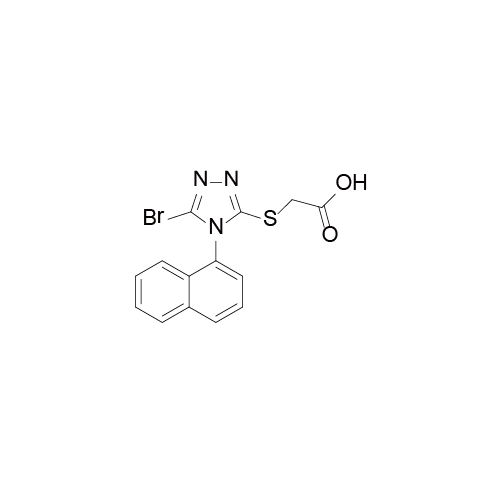
Lesinurad 雷西纳德
Lesinurad于2015年12月22日获得了美国食品药品监督管理局(FDA)的上市批准,之后于2016年2月18日获欧洲药品管理局(EMA)批准上市。该化合物早前由Ardea公司研发。而阿斯利康于2012年收购了该公司,进而获得了该产品。该药美国地区及欧盟地区的商品名为Zurampic®.
Lesinurad是全球首个获批的尿酸盐重吸收转运子(URAT1)抑制剂,联合黄嘌呤氧化酶抑制剂治疗高尿酸血症相关的痛风。
Zurampic®是口服用片剂,规格是200毫克。推荐剂量是200毫克一天一次,与别嘌醇或者非布司他联用。
基本信息
更新时间:2017-04-21
- 药品名称:
- Lesinurad 雷西纳德
- 研发代码:
- RDEA-594 RDEA594 sodium
- 商品名称:
- Zurampic®
- 作用机制:
- Uric acid transporter 1 (URAT1) inhibitors Organic anion transporter 4 (OAT4) inhibitors
- 适应症:
- 高钙血症,痛风
- 研发阶段:
- 批准上市
- 研发公司:
- 阿斯利康 (原研)
- 年销售额:
- ATC号:
主要国家或地区批准情况
更新时间:2016-03-10
| 批准日期 | NDA号 | 商品名 | 适应症 | 剂型 | 规格 | 公司 | 审查类别 |
|---|---|---|---|---|---|---|---|
| 2015-12-22 | NDA 207988 | Zurampic | 痛风, 高尿酸血症 | 片剂,薄膜包衣片 | 200 mg | 阿斯利康 |
| 批准日期 | NDA号 | 商品名 | 适应症 | 剂型 | 规格 | 公司 | 审查类别 |
|---|---|---|---|---|---|---|---|
| 2016-02-18 | EMEA/H/C/003932 | Zurampic | 高尿酸血症, 痛风 | 片剂,薄膜包衣片 | 200 mg | 阿斯利康 |
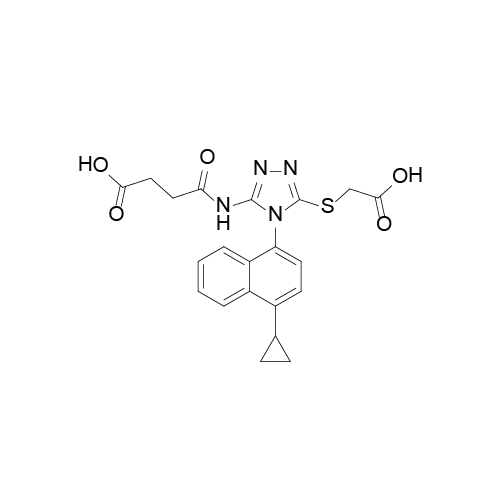
化学信息
更新时间:2015-12-24
专利信息
更新时间:2016-01-06
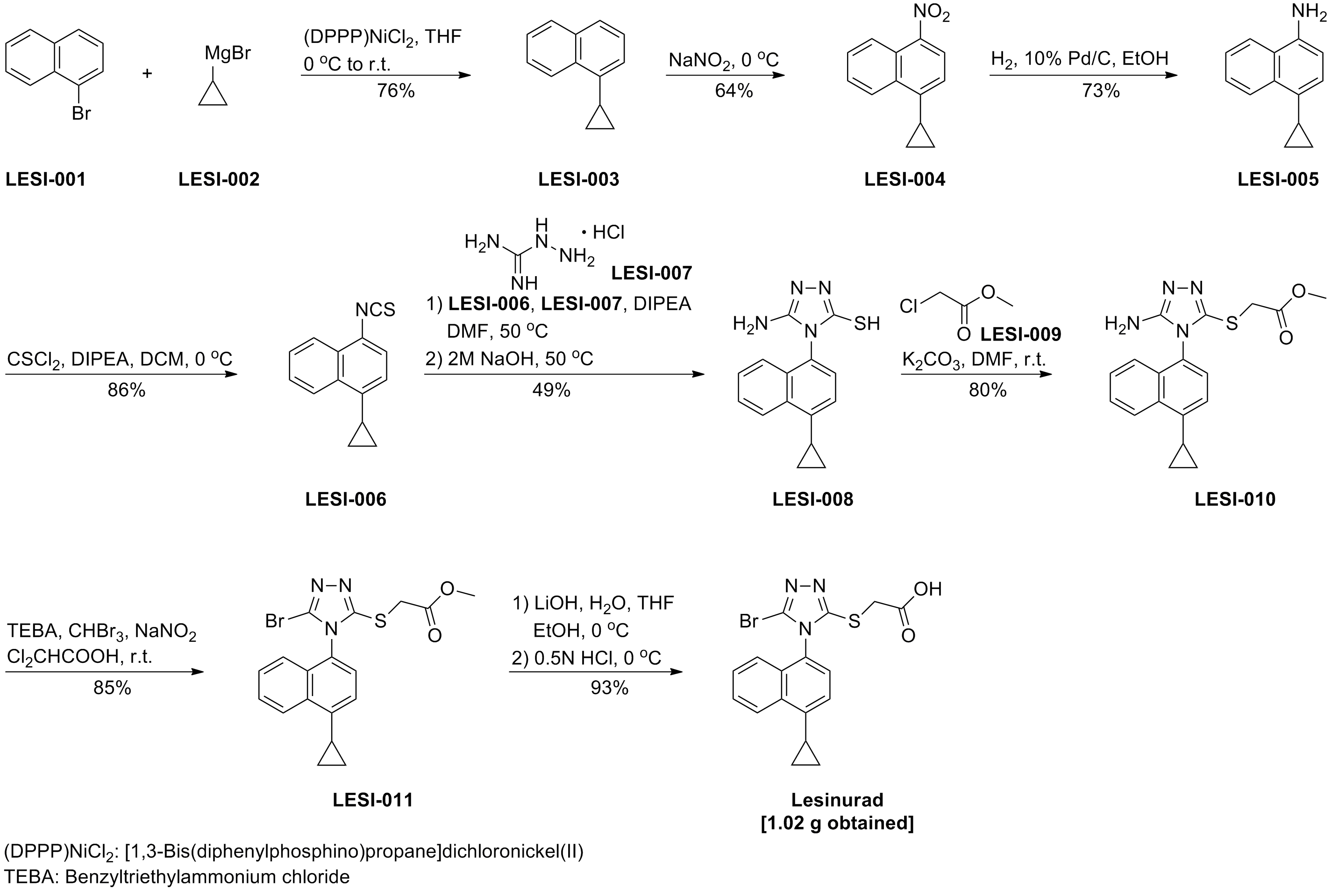
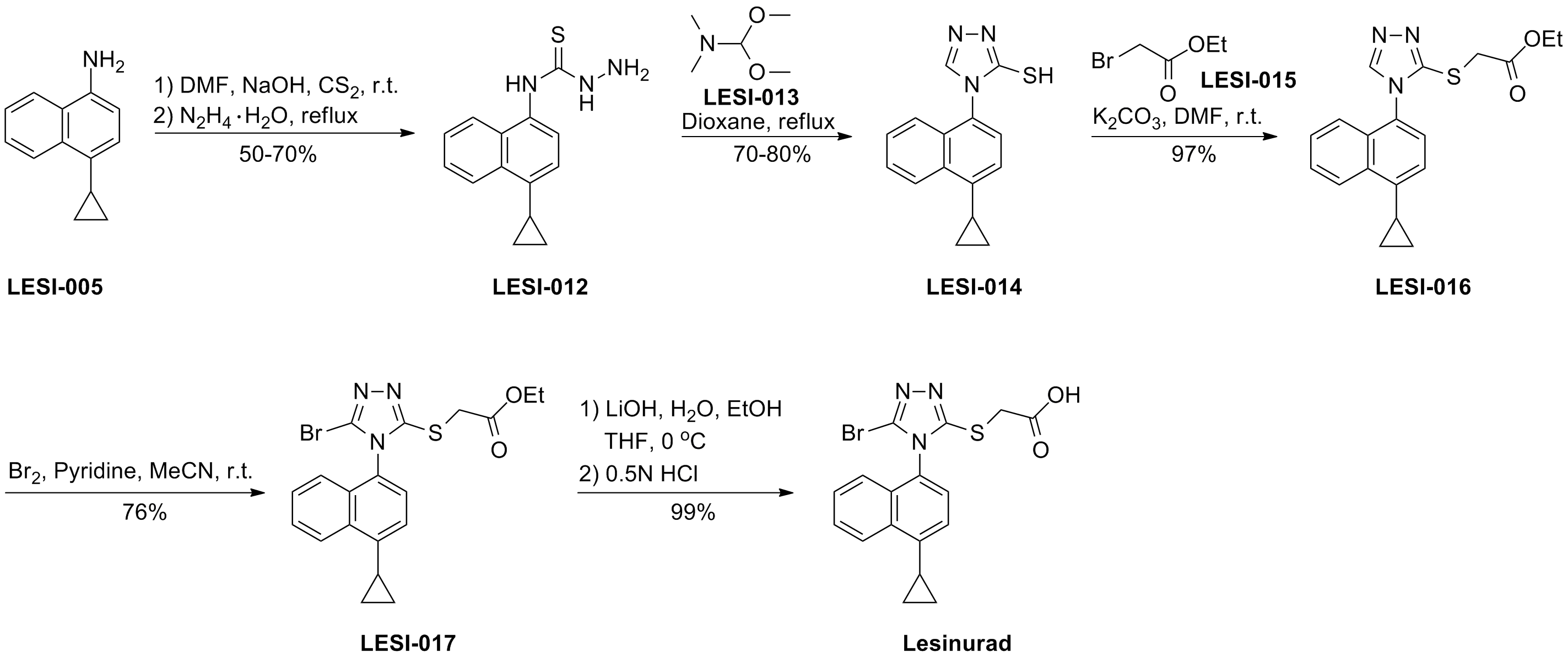
化学合成路线及相关杂质
更新时间:2017-04-18
1. WO2015054960A1.
Clinical Trial 信息
NCT号
试验标题
适应症
阶段
状态
Lesinurad and Febuxostat Combination Extension Study in Gout
Gout
Active not recruiting
Phase Ⅲ
Lesinurad and Allopurinol Combination Extension Study in Gout
Gout
Active not recruiting
Phase Ⅲ
Combination Treatment Study in Subjects With Tophaceous Gout With Lesinurad and Febuxostat
Tophaceous Gout
Completed
Phase Ⅲ
Lesinurad Monotherapy in Gout Subjects Intolerant to Xanthine Oxidase Inhibitors
Gout
Completed
Phase Ⅲ
Clinical Trial 信息
更新时间:2016-09-28
NCT号
试验标题
适应症
阶段
状态
Lesinurad and Febuxostat Combination Extension Study in Gout
Gout
Active not recruiting
Phase Ⅲ